Dehydration of 4 methylcyclohexanol mechanism – The dehydration of 4-methylcyclohexanol, a fundamental organic reaction, offers a compelling exploration into the intricacies of chemical transformations. This in-depth analysis delves into the mechanism, factors, regioselectivity, applications, and safety considerations associated with this versatile process, providing a comprehensive understanding of its significance in organic chemistry.
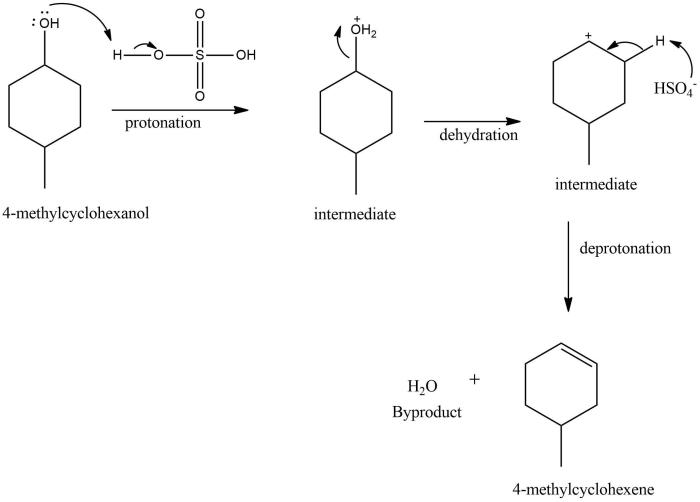
The dehydration reaction of 4-methylcyclohexanol, a crucial step in the synthesis of various organic compounds, involves the removal of a water molecule to form 1-methylcyclohexene. This transformation is governed by a detailed step-by-step mechanism, influenced by factors such as temperature, acid catalysts, and solvent polarity.
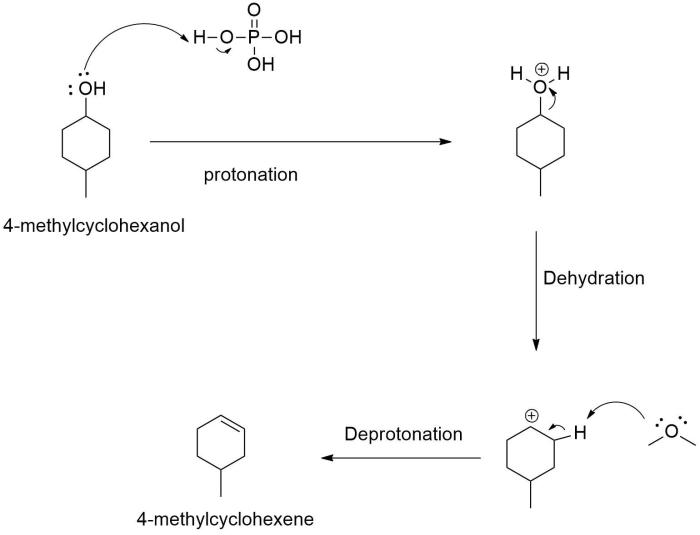
Mechanism of Dehydration of 4-Methylcyclohexanol
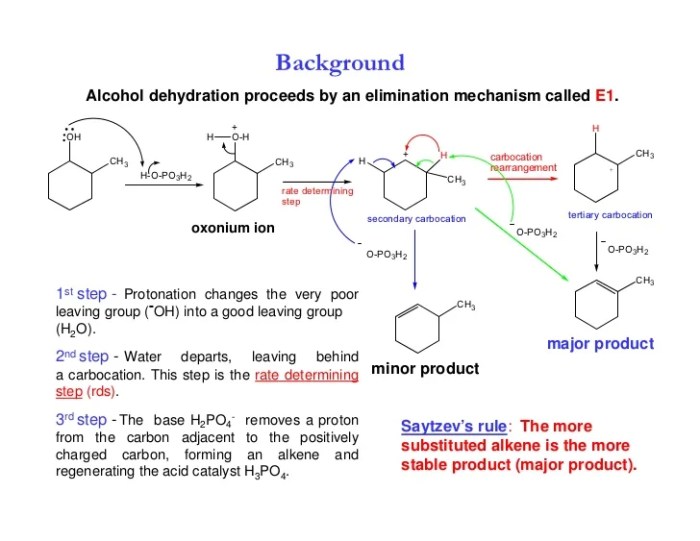
The dehydration of 4-methylcyclohexanol to form 1-methylcyclohexene is a classic example of an elimination reaction. The reaction is catalyzed by an acid, such as sulfuric acid or phosphoric acid, and proceeds through a carbocation intermediate.
The first step in the reaction is the protonation of the alcohol group by the acid. This forms a positively charged oxonium ion, which is a good leaving group. The oxonium ion then collapses, expelling a water molecule and forming a carbocation.
The carbocation is then attacked by a base, such as pyridine or triethylamine. This forms a double bond between the carbon atom that was originally bonded to the alcohol group and the carbon atom that was originally bonded to the hydrogen atom.
The product of the reaction is 1-methylcyclohexene.
Factors Affecting the Dehydration Reaction
The dehydration of 4-methylcyclohexanol is affected by a number of factors, including temperature, acid catalyst, and solvent polarity.
Temperature
The rate of the dehydration reaction increases with increasing temperature. This is because the higher the temperature, the more energy the molecules have, and the more likely they are to overcome the activation energy for the reaction.
Acid Catalyst
The acid catalyst plays an important role in the dehydration reaction. The acid protonates the alcohol group, making it a better leaving group. The acid also helps to stabilize the carbocation intermediate.
Solvent Polarity
The solvent polarity also affects the dehydration reaction. Polar solvents, such as water, solvate the ions in the reaction, making them less reactive. Nonpolar solvents, such as dichloromethane, do not solvate the ions as well, making them more reactive.
Regioselectivity of the Dehydration Reaction
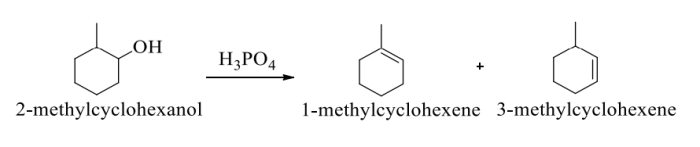
The dehydration of 4-methylcyclohexanol can produce two possible products: 1-methylcyclohexene and 2-methylcyclohexene. However, the reaction preferentially forms 1-methylcyclohexene. This is because the carbocation intermediate is more stable when it is formed on the carbon atom that is bonded to the methyl group.
The stability of the carbocation intermediate is due to two factors: steric effects and electronic effects.
Steric Effects
The methyl group on the carbon atom that is bonded to the alcohol group hinders the approach of the base to the other carbon atom. This makes it more difficult to form the 2-methylcyclohexene product.
Electronic Effects, Dehydration of 4 methylcyclohexanol mechanism
The methyl group on the carbon atom that is bonded to the alcohol group donates electrons to the carbocation intermediate. This makes the carbocation intermediate more stable.
Applications of the Dehydration Reaction
The dehydration of 4-methylcyclohexanol is a versatile reaction that has a number of applications in organic chemistry.
- The reaction can be used to produce 1-methylcyclohexene, which is a valuable intermediate in the synthesis of other organic compounds.
- The reaction can also be used to remove water from other organic compounds.
- The reaction is also used in the production of biodiesel.
Safety Considerations: Dehydration Of 4 Methylcyclohexanol Mechanism
The dehydration of 4-methylcyclohexanol is a hazardous reaction that should be carried out with caution.
- The acid catalyst is corrosive and can cause burns.
- The reaction produces water, which can cause the reaction mixture to boil over.
- The products of the reaction are flammable.
To avoid these hazards, it is important to take the following safety precautions:
- Wear gloves and eye protection when handling the acid catalyst.
- Carry out the reaction in a well-ventilated area.
- Use a reflux condenser to prevent the reaction mixture from boiling over.
- Dispose of the products of the reaction properly.
Popular Questions
What is the driving force behind the dehydration of 4-methylcyclohexanol?
The dehydration reaction is driven by the formation of a more stable alkene product, 1-methylcyclohexene, which is thermodynamically favored over the starting alcohol.
How does temperature affect the rate of dehydration?
Temperature has a significant impact on the reaction rate, with higher temperatures leading to faster dehydration due to increased molecular energy and collision frequency.
What role do acid catalysts play in the dehydration process?
Acid catalysts, such as sulfuric acid or phosphoric acid, protonate the alcohol group, making it a better leaving group and facilitating the elimination of water.