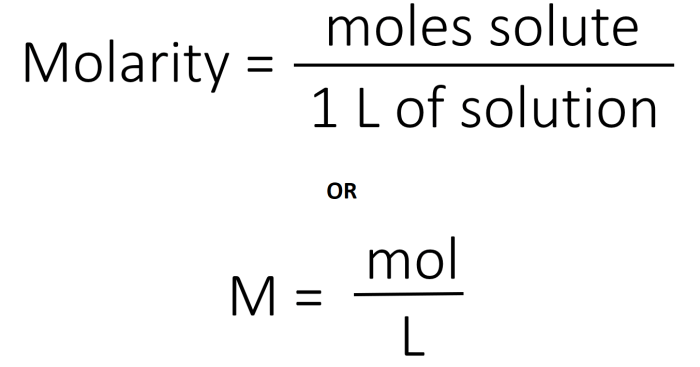Stoichiometry problems chem worksheet 12 2 – Embark on a comprehensive exploration of stoichiometry problems with Chem Worksheet 12.2. This in-depth resource provides a structured and engaging approach to understanding the fundamental principles and applications of stoichiometry. Through a series of well-crafted exercises and thought-provoking questions, learners will gain a deep comprehension of this essential chemical concept.
Delving into the intricacies of stoichiometry, this worksheet guides learners through the concepts of mole calculations, limiting reactants, gas reactions, solution reactions, and real-world applications. Each section is meticulously crafted to build a strong foundation and foster critical thinking skills.
Stoichiometry Basics: Stoichiometry Problems Chem Worksheet 12 2

Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It is a fundamental concept in chemistry that helps us understand the composition of compounds, predict the products of reactions, and determine the amount of reactants and products involved in a reaction.
The law of conservation of mass states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products. This law is based on the principle that matter cannot be created or destroyed, only rearranged.
Balanced chemical equations represent chemical reactions in a way that shows the mole ratios of the reactants and products. For example, the following equation shows the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation shows that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
Mole Calculations
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains exactly 6.022 × 10^23 elementary entities. The mole can be used to convert between the mass and the number of particles of a substance.
To convert between mass and moles, we use the following formula:
moles = mass / molar mass
where molar mass is the mass of one mole of a substance in grams.
For example, to convert 100 grams of sodium chloride to moles, we would use the following formula:
moles = 100 g / 58.44 g/mol = 1.71 mol
Limiting Reactants and Excess Reactants
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, while the excess reactant is the reactant that is left over after the reaction is complete.
To determine the limiting reactant, we compare the mole ratios of the reactants to the stoichiometric coefficients in the balanced chemical equation.
For example, consider the following reaction:
2H₂ + O₂ → 2H₂O
If we have 2 moles of hydrogen and 1 mole of oxygen, we can compare the mole ratios to the stoichiometric coefficients:
H₂: 2 moles / 2 = 1
O₂: 1 mole / 1 = 1
The mole ratio of hydrogen is 1, and the mole ratio of oxygen is also 1. This means that the mole ratios of the reactants are equal to the stoichiometric coefficients, so neither reactant is limiting.
Stoichiometry in Gas Reactions
Stoichiometry can be used to calculate the volumes of gases involved in a reaction. The ideal gas law is a mathematical equation that relates the pressure, volume, temperature, and number of moles of a gas.
The ideal gas law is:
PV = nRT
where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m³)
- n is the number of moles of the gas
- R is the ideal gas constant, which is 8.314 J/mol·K
- T is the temperature of the gas in kelvins (K)
Stoichiometry in Solution Reactions
Stoichiometry can be used to calculate the concentrations of solutions. The concentration of a solution is a measure of the amount of solute that is dissolved in a given volume of solvent.
The most common units of concentration are molarity (M), normality (N), and mass percent (%).
- Molarity is defined as the number of moles of solute per liter of solution.
- Normality is defined as the number of equivalents of solute per liter of solution.
- Mass percent is defined as the mass of solute per 100 grams of solution.
Stoichiometry Applications, Stoichiometry problems chem worksheet 12 2
Stoichiometry has a wide range of applications in various fields, including:
- Environmental chemistry: Stoichiometry is used to calculate the amount of pollutants released into the environment and to develop methods for reducing pollution.
- Industrial chemistry: Stoichiometry is used to optimize chemical processes and to design new products.
- Medicine: Stoichiometry is used to calculate the dosages of drugs and to develop new drug therapies.
FAQ Summary
What is the significance of stoichiometry in chemistry?
Stoichiometry plays a fundamental role in chemistry as it enables the determination of the quantitative relationships between reactants and products in chemical reactions. By understanding stoichiometry, chemists can predict the amounts of reactants and products involved in a given reaction, ensuring efficient and accurate experimentation.
How can I improve my understanding of stoichiometry problems?
To enhance your comprehension of stoichiometry problems, it is essential to practice regularly. Utilize resources such as Chem Worksheet 12.2, engage in discussions with peers and instructors, and seek additional problem sets to challenge your understanding. Consistent practice will strengthen your problem-solving skills and deepen your conceptual knowledge.
What are some real-world applications of stoichiometry?
Stoichiometry finds numerous applications in various fields, including environmental chemistry, industrial chemistry, and medicine. In environmental chemistry, stoichiometry is employed to determine the amounts of pollutants released into the environment and to develop strategies for their mitigation. In industrial chemistry, stoichiometry is crucial for optimizing production processes and ensuring product quality.
In medicine, stoichiometry plays a vital role in drug development and dosage calculations, ensuring the safe and effective administration of medications.